
Vendor Auditing

Vendor Quality Audits for GxP environments
In addition to our IT specialism with audit experience since 2004, we perform vendor quality audits for GCP, GMP, GLP and GPvP, we can deliver, qualification, routine or for cause vendor audits on your suppliers from CROs, to laboratories and IT supplier, we can help you assess your vendors capabilities, quality management, compliance and suitability.
IT / CSV Auditing

Quality Assurance Auditing
With our years of experience in IT Quality Assurance and working with people from all levels of global pharmaceutical organisations from the CEO to the factory floor we can assist you with our varied range of IT Quality Assurance Services. Whether you need help and support with a specific audit / audits, or outsource your IT QA audit schedule.
Validation
Computer Systems Validation
Based on our knowledge of GAMP and Industry Best Practices for Computerised System Validation we provide risk-based validation services for small and large systems alike. We have experience in validating computer systems across the GxP spectrum from Laboratory Information Management systems, Manufacturing Materials Management and Dispensing Systems to Clinical Trial Management, Regulatory Document Management Submissions (CTD / eCTD) and Pharmacovigilance systems as well as infrastructure services including IT Service Desks, Data Centres and Networks.
Data Integrity and ERES
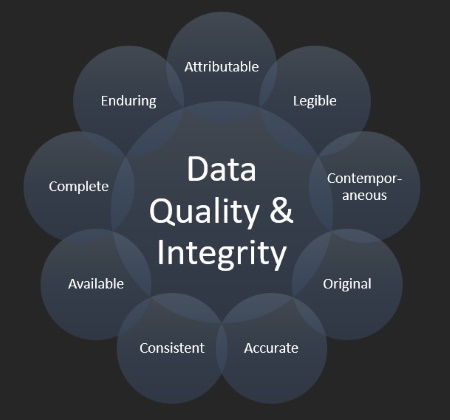
Data Integrity, Electronic Records and Signatures, CFR 21 Part 11
We can help ensure your systems comply with the latest guidance and regulations for Data Integrity, Electronic Records and Signatures. Whether it is working with your own internal Data Integrity and ERES Compliance and Assessment process or, through our own ERES and 21 CFR Part 11 Control Framework and Data Integrity support.
We are a UK based Pharmaceutical Consultancy specialising in Data Integrity, Computerised System Validation and IT Quality Assurance services.
TS CSV QA’s mission is to deliver pragmatic fit for purpose solutions to our clients to support technological implementation and innovation in the Pharmaceutical and Healthcare sectors.
Founded by Trev Simmons, Trev is a Fellow of the Research Quality Association, and an active member of the association, and is currently Chair of the Digital Information Governance and Information Technology (DIGIT) Committee as well as the Cross-GxP Working Party on Data Integrity.
